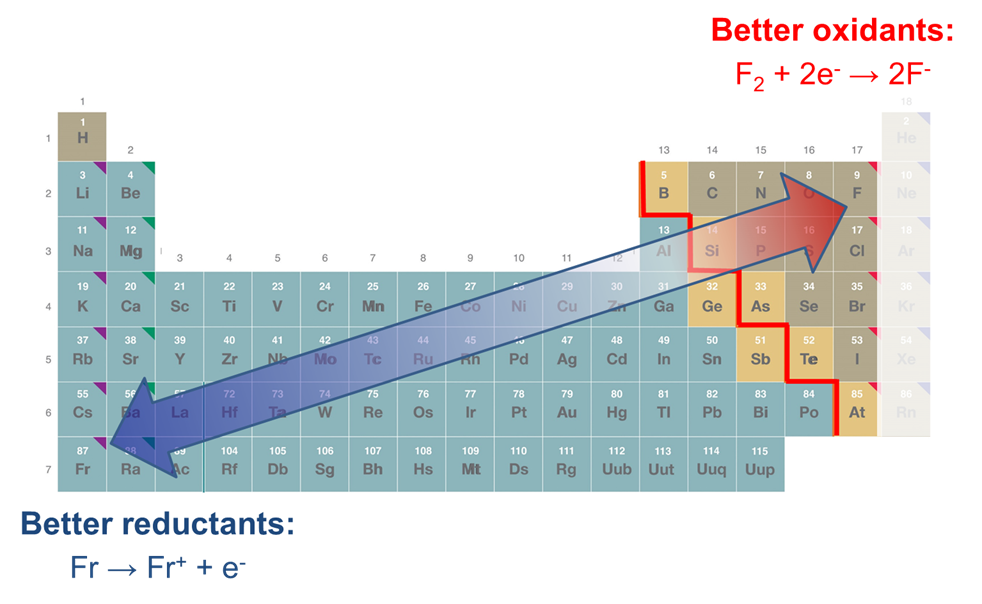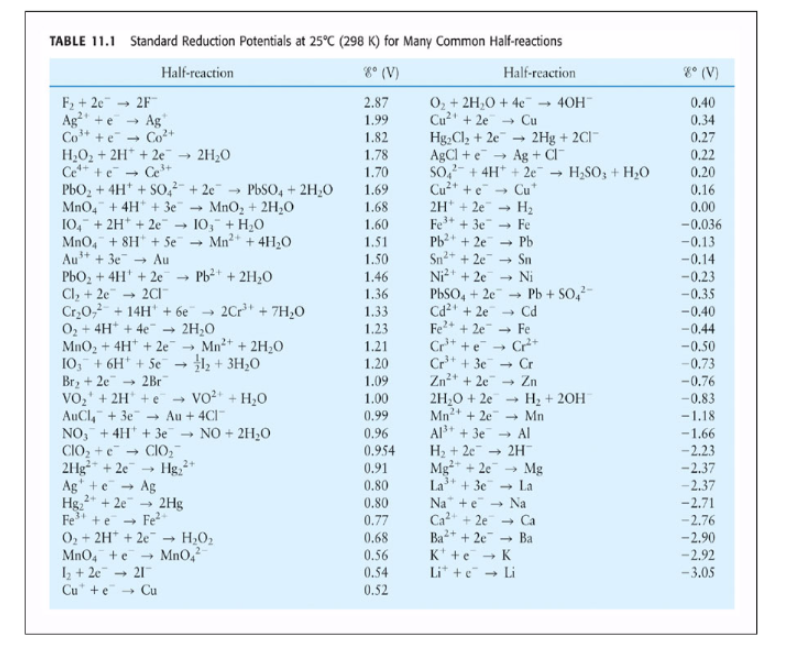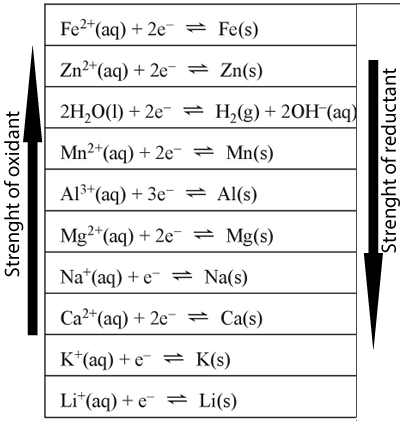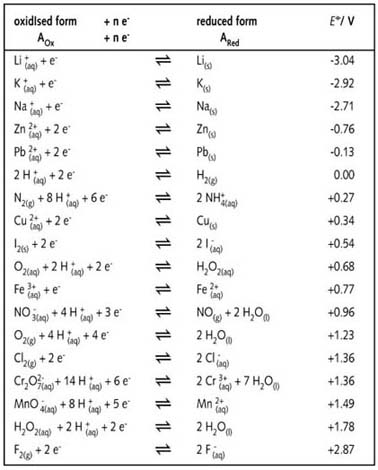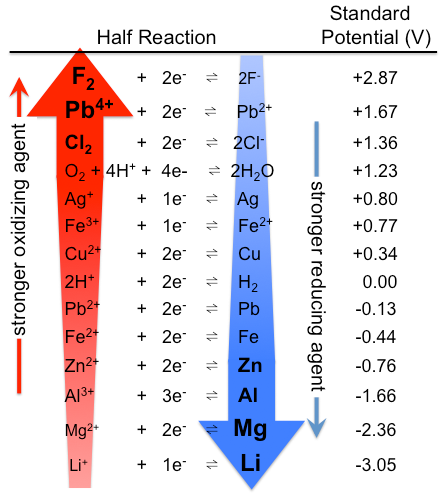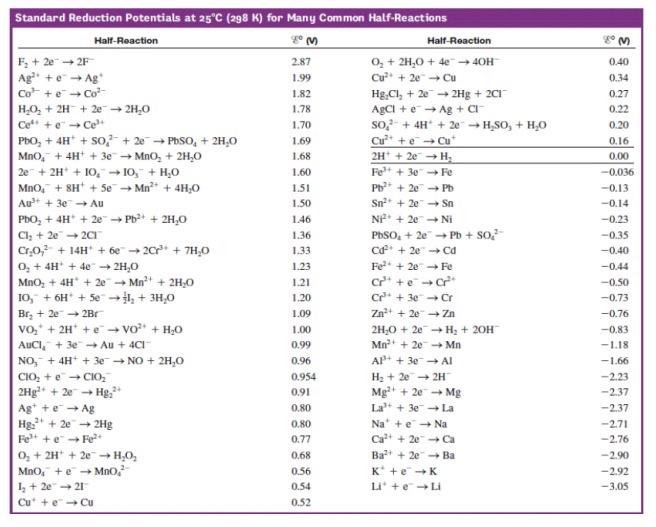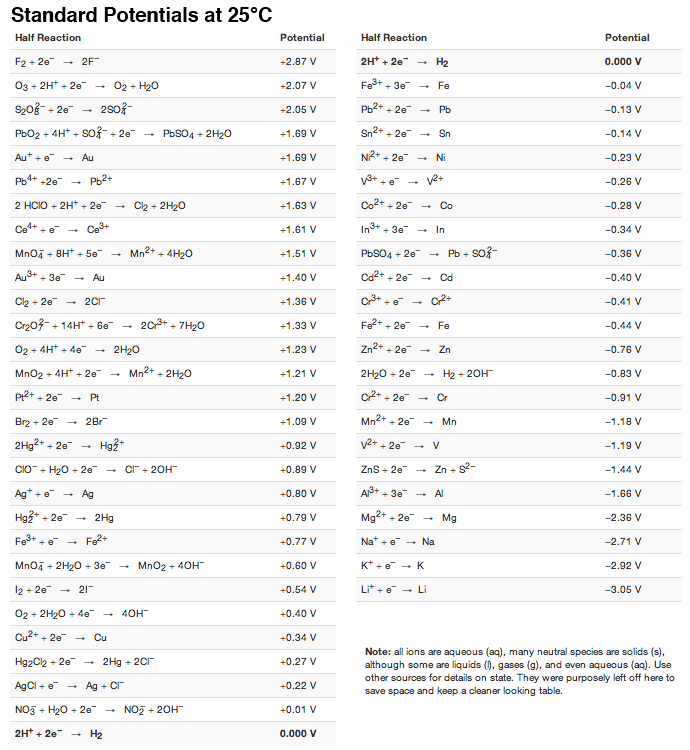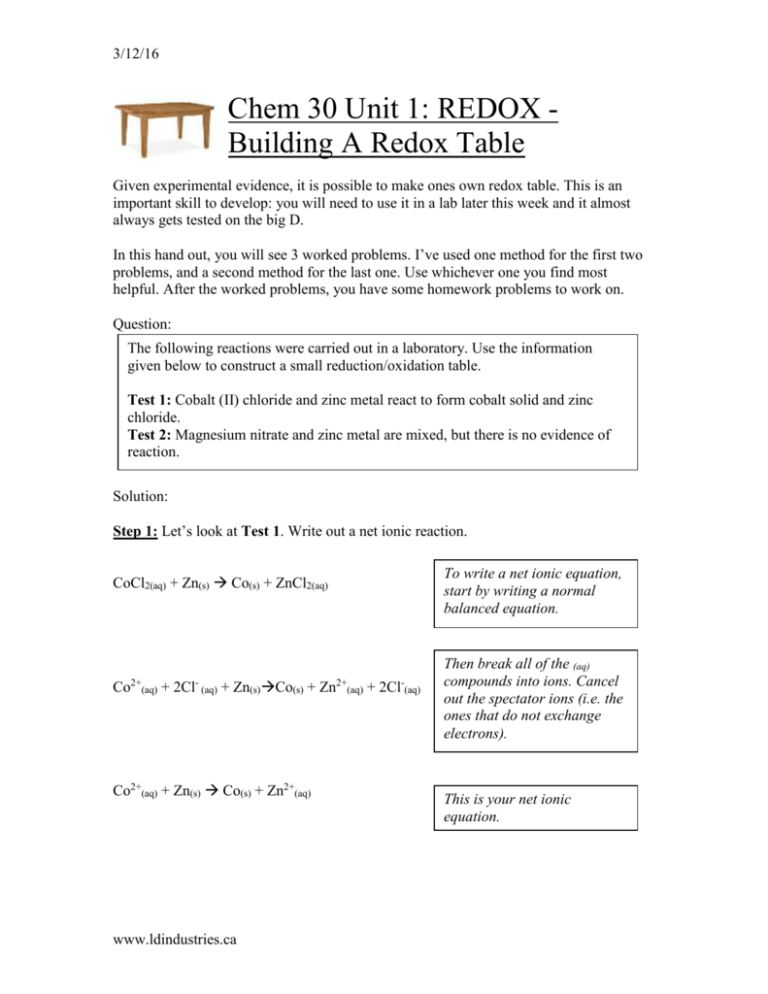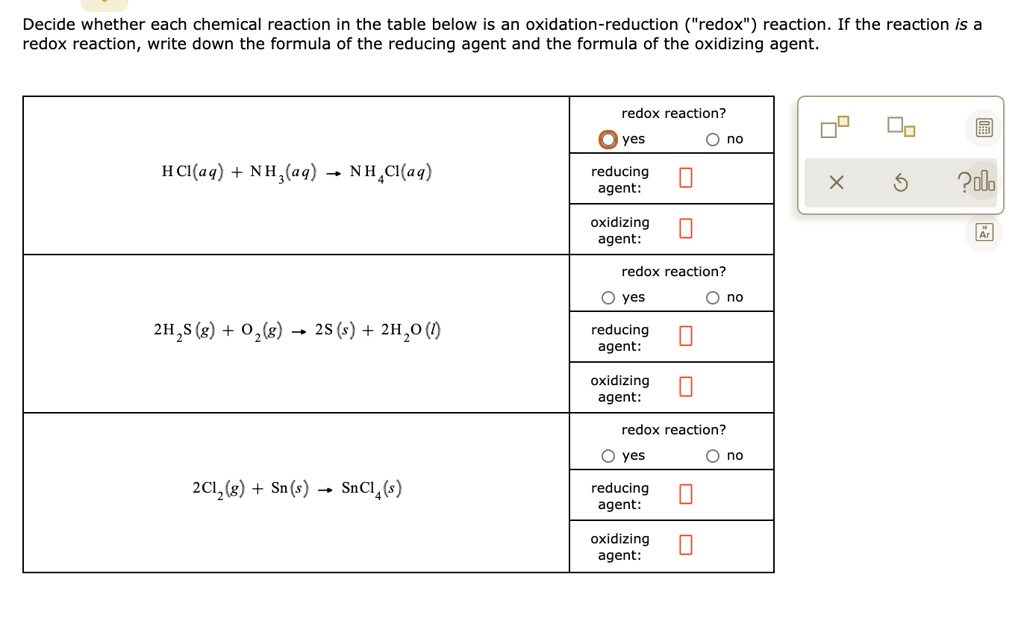
SOLVED: Decide whether each chemical reaction in the table below is an oxidation-reduction "redox" reaction. If the reaction is a redox reaction, write down the formula of the reducing agent and the

electrochemistry - How to calculate whether a redox reaction is spontanuous? - Chemistry Stack Exchange

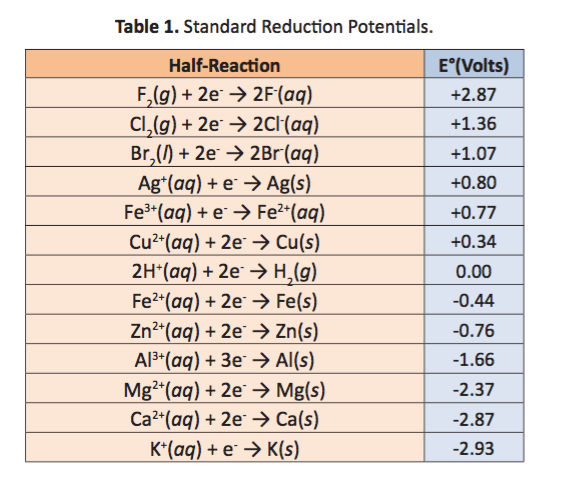
Table 1 from Tungsten's redox potential is more temperature sensitive than that of molybdenum. | Semantic Scholar

OneClass: Use the Standard Reduction Table to write net ionic equations for the extensive redox react...