
Auditing Organization (AO) versus Notified Body (NB) versus Registrar. What's the difference? – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog

TÜV SÜD - Healthcare & Medical Devices on LinkedIn: #tüvsüd #mhs #notifiedbody #notifiedbody2443 #denmark #mdr
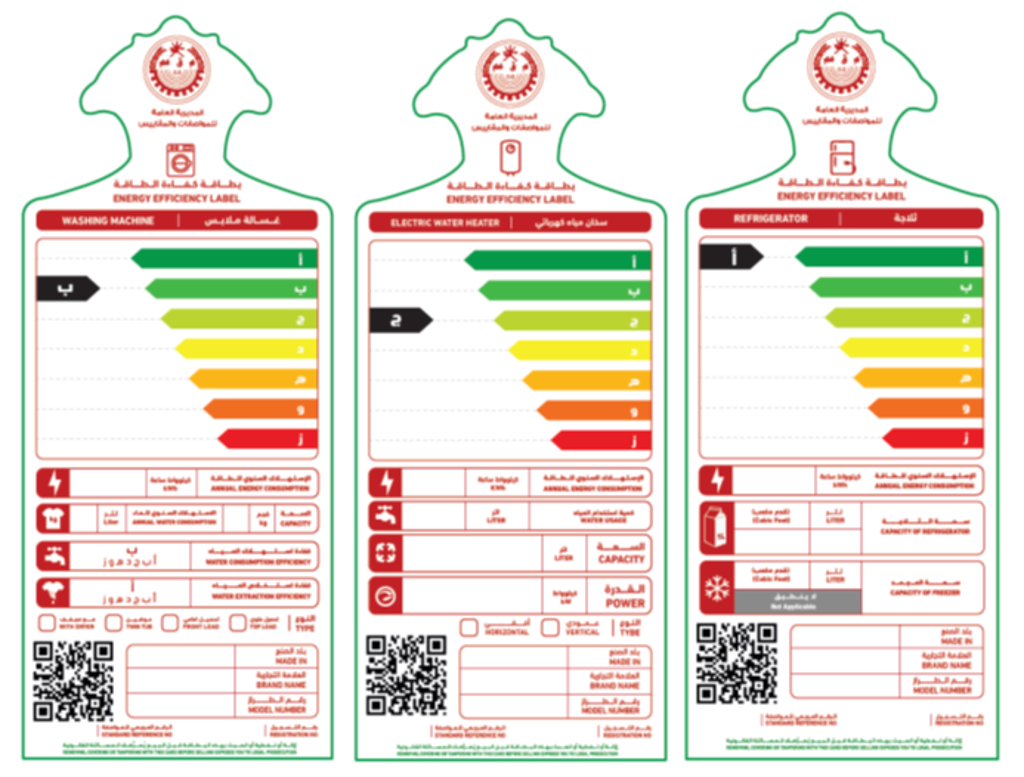
TÜV Rheinland Designated as Omani Energy Efficiency Notified Body for Refrigerators, Washing Machines and Water Heaters | TÜV Rheinland
UK Medical Devices Regulations: TÜV Rheinland is now a UK Approved Body according to UK MDR | TÜV Rheinland

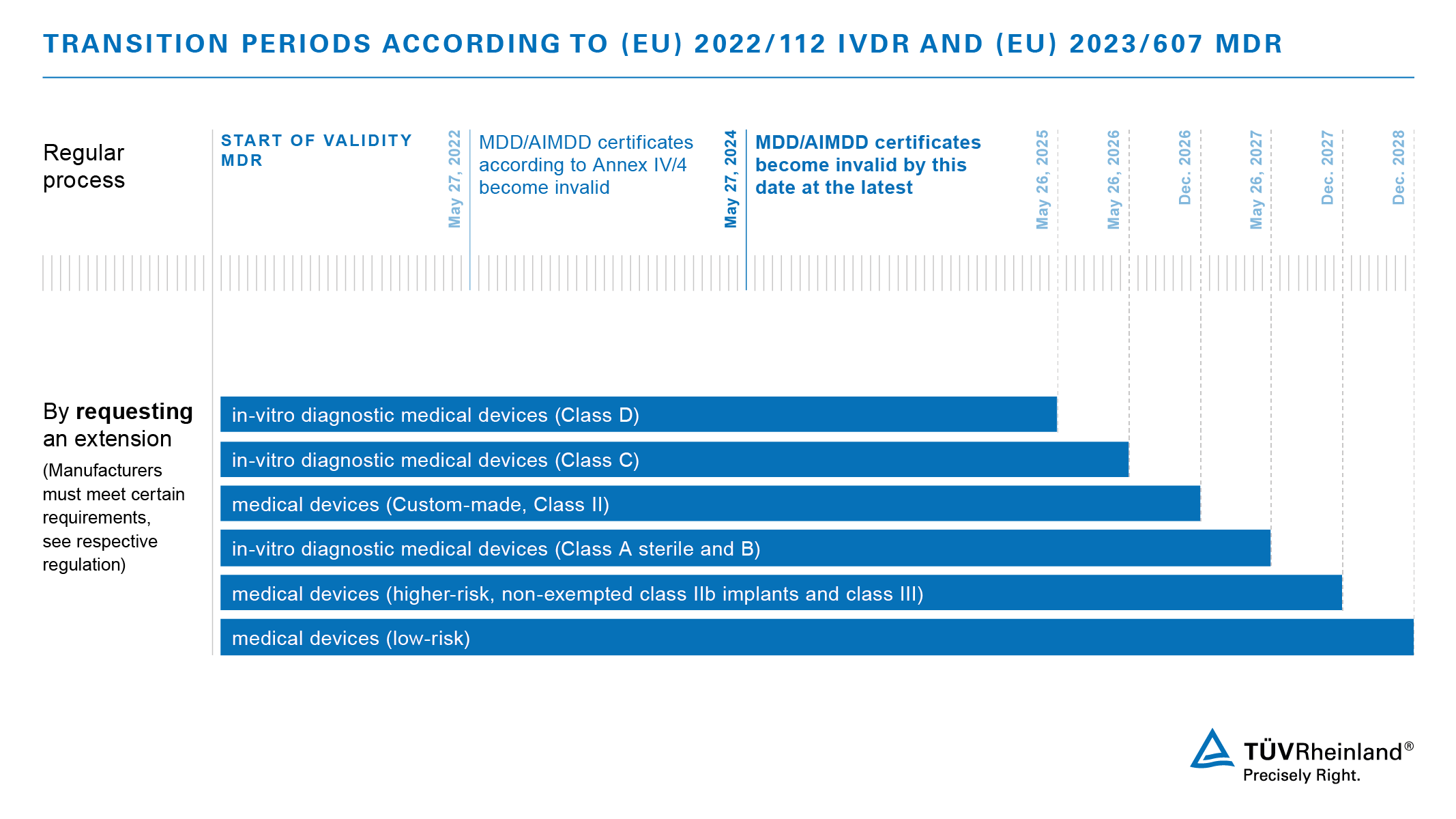
/tuv-rheinland-ivdr-visual-1-en_core_1_x.png)








![How to approach your Notified Bodies? [Dr. Royth von Hahn - TÜV SÜD] How to approach your Notified Bodies? [Dr. Royth von Hahn - TÜV SÜD]](https://podcast.easymedicaldevice.com/wp-content/uploads/2021/11/155_NB_upgrade_Royth_von_hahn_2x3_LOW.jpg)








